Battery Chemistry
Older batteries were mostly based on rechargeable lead-acid or non-rechargeable alkaline chemistries, with nominal voltages in increments of 2.10 - 2.13 and 1.5 Volts respectively, each representing one individual electrochemical cell.
New special battery chemistries have strained older naming conventions. Rechargeable NiCd (Nickel Cadmium) and NiMH (Nickel Metal Hydride) typically output 1.25 V per cell. Some devices may not operate properly with these cells, given the 16% reduction in voltage, but most modern ones handle them well. Conversely, lithium-ion rechargeable batteries output 3.7 V per cell, 23% higher than a pair of alkaline cells (3 V), which they are often designed to replace. Non-rechargeable lithium-chemistry batteries, which provide exceptionally high energy density, produce about 1.5 V per cell and are thus similar to alkaline batteries.
Many new battery sizes refer to both the batteries' size and chemistry, while older names do not. This summary is only for types relating to battery "sizes".
Primary Battery Chemistries
| Chemistry | Cell Voltage | Energy Density (MJ/kg) | Elaboration |
|---|---|---|---|
| Zinc–carbon | 1.5 | 0.13 | Inexpensive. |
| Zinc chloride | 1.5 | Also known as "heavy duty", inexpensive. | |
| alkaline (zinc–manganese dioxide) |
1.5 | 0.4-0.59 | Moderate energy density. Good for high and low drain uses. |
| oxy nickel hydroxide (zinc-manganese dioxide/oxy nickel hydroxide) |
1.7 | Moderate energy density. Good for high drain uses. |
|
| Lithium (lithium–copper oxide) Li–CuO |
1.7 | No longer manufactured. Replaced by silver oxide (IEC-type "SR") batteries. |
|
| Lithium (lithium–iron disulfide) LiFeS2 |
1.5 | Expensive. Used in 'plus' or 'extra' batteries. |
|
| Lithium (lithium–manganese dioxide) LiMnO2 |
3.0 | 0.83 – 1.01 | Expensive. Only used in high-drain devices or for long shelf life due to very low rate of self discharge. 'Lithium' alone usually refers to this type of chemistry. |
| Mercury oxide | 1.35 | High drain and constant voltage. Banned in most countries because of health concerns. |
|
| Zinc–air | 1.35 – 1.65 | 1.59[1] | Mostly used in hearing aids. |
| Silver oxide (silver-zinc) |
1.55 | 0.47 | Very expensive. Only used commercially in 'button' cells. |
Rechargeable Battery Chemistries
| Chemistry | Cell Voltage | Energy Density (MJ/kg) | Comments |
|---|---|---|---|
| NiCd | 1.2 | >0.14 | Inexpensive. High/low drain, moderate energy density. Can withstand very high discharge rates with virtually no loss of capacity. Moderate rate of self discharge. Reputed to suffer from memory effect (which is alleged to cause early failure). Environmental hazard due to Cadmium - use now virtually prohibited in Europe. |
| Lead Acid | 2.2 | >0.14 | Moderately expensive. Moderate energy density. Moderate rate of self discharge. Higher discharge rates result in considerable loss of capacity. Does not suffer from memory effect. Environmental hazard due to Lead. Common use - Automobile batteries |
| NiMH | 1.2 | >0.36 | Cheap. Not usable in higher drain devices. Traditional chemistry has high energy density, but also a high rate of self-discharge. Newer chemistry has low self-discharge rate, but also a ~25% lower energy density. Very heavy. Used in some cars. |
| Lithium ion | 3.6 | >0.46 | Very expensive. Very high energy density. Not usually available in "common" battery sizes (but see RCR-V3 for a counter-example). Very common in laptop computers, moderate to high-end digital cameras and camcorders, and cellphones. Very low rate of self discharge. Volatile: Chance of explosion if short circuited, allowed to overheat, or not manufactured with rigorous quality standards. |
| Lithium Cobalt Oxide (LiCoO2) | 3.6 | >0.72 | High specific energy. Relatively short life span, Low thermal stability and limited load capabilities (specific power). Should not be charged and discharged at a current higher than its C-rating |
| Lithium Iron Phosphate (LiFePO4) | 3.3 | >0.32 | Good electrochemical performance with low resistance. High discharging current. Cold temperature reduces performance and elevated storage temperature shortens the service life. Limited “C-rate” of around 1C, which means they take a long time to charge. Excellent safety and long life span. Moderate specific energy and elevated self-discharge. |
| Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) | 3.7 | >0.54 | C-rate” of this chemistry can range from 1-5C. Higher energy density with lower cost, long cycle life. Can have either a high specific energy or high specific power, they cannot, however, have both properties. Very low self-heating rate. |
| Lithium Manganese Oxide (LiMn2O4) | 3.8 | >0.36 | High thermal stability and enhanced safety, but the cycle and calendar life are limited. Low internal cell resistance enables fast charging and high-current discharging. Can be discharged at currents of 20–30A with moderate heat buildup. |
| Lithium Titanate (Li2TiO3) | 2.4 | >0.23 | Expensive. Excels in safety, low-temperature performance. Long cycle life: > 3000-7000 cycles. Can be fast charged and delivers a high discharge current of 10C. Cycle count is said to be higher than that of a regular Li-ion. Thermal stability under high temperature is also better than other Li-ion systems. |
Typical specific energy of lead-, nickel-, and lithium-based batteries
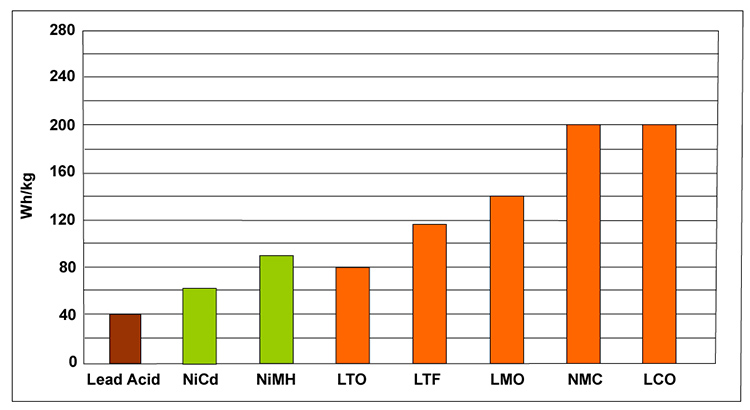
LFP - Lithium Iron Phosphate
LCO - Lithium Cobalt Oxide
LMO - Lithium Magnesium Oxide
NMC - Lithium Nickel Manganese Cobalt Oxide
LTO - Lithium Titanate
Unsure What Battery Chemistry to Select for Your Application?
Confused by the maze of battery chemistries and their implications? Don't compromise your project with the wrong cell choice. Consult with our experts to select the right battery for your specific needs.
Request a Quote Request Design Support Request More Information


