
Battery Packs for Medical DevicesWhere Quality is put to the test!
If innovation and leading edge technologies in powering your medical device is in your product plan, Epec Engineered Technologies is the single source solution that can quickly and safely get you from concept to production. We possess the staff and expertise to design and develop your power management system that meets all domestic and international safety requirements (IEC 60601 or UL 2601 and CAN/CSA-C22.2 60601).
Through the proven Epec systems, lean operating principles and tools, we work to hone a competitive edge while remaining focused on the needs and expectations of our customers. While portability has made medical devices more practical, the true measure of effectiveness is how efficiently the device can be powered to create the longest run time possible for the patient.
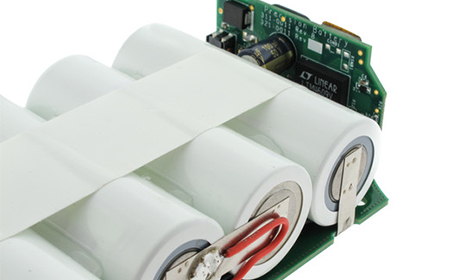

Engineering Resource for Medical Device OEM’s
As a diversified manufacturer and cutting-edge engineering resource for medical device OEM’s, our capabilities start with:
- Design for manufacturing and cost management
- Quality and regulatory assurance
- Product and process verification
- Product safety integration
- FDA compliant documentation
- Post-production service and support
Regulatory Approval
Regulatory institutions are concerned that device manufacturers do not place sufficient importance on battery management and use. Every medical device is different, to include how the profile is used, and the fact that it is subjected to numerous environmental conditions which require the OEM to partner with an experienced battery partner.
With that in mind, the U.S. FDA requires registered manufacturers to keep tight control over their approved vendor list (AVL). We leverage our existing relationships and experience with the FDA as they regularly visit our facility as part of our current agreements with medical device manufactures.
While the FDA oversees the approval of medical devices, battery packs, and other electronics are also subject to approvals from UL, IATA, DOT and other regulatory agencies. Here at Epec we have over 30 years of experience in designing and manufacturing products that exceeds these requirements as testing and certification for medical devices are focused on demonstrating risk management. It should be noted that this gives companies the option of following the IEC’s question-based approach or a registration audit approach developed by the International Organization for Standardization (ISO). UL 60601 is the harmonized standard for IEC 60601 and provides another pathway to certification.
Battery Pack Traceability
Quality and reliability have always been Epec’s highest priority when designing and manufacturing of custom battery packs. While inspection all components , and a development of a leading edge test protocol and fixtures are standard process, medical devices require that we go miles beyond the traditional quality measures.
We serialize every battery pack after test with several pieces of information, including the serial number, the day it was tested, by whom it was tested and the diagnostic results. This data is not only stored on our cloud based computer system, but it is written back to the chip on the device itself. This allows us to track lot codes of components and troubleshoot individual units based upon their diagnostic review.
Battery Packs for Class III Medical Device Applications
The U.S. Food and Drug Administration’s (FDA) list of medical devices stretches to nearly 6,000 products. These products are categorized in three classes:
- Class I medical devices, which are subject to the least regulatory control, present minimal potential for harm to the user, and are often simpler in design than Class II or Class III devices.
- Class II medical devices, which are subject to special controls, often including labeling requirements, mandatory performance standards, and post-market surveillance.
- Class III medical devices, which are subject to the most stringent controls because they often support or sustain human life, are importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury.
While not all cell manufacturers will support Class III Medical Device applications, Epec Engineered Technologies has experience in designing and manufacturing battery packs that can satisfy the requirements for all three classes of medical device products. Everything from managing the testing processes through UL, meeting the IEC 60601 Standard, making sure that you have multiple level of safety redundancy or working with the FDA when they tour our facility, our team has the expertise to smoothly guide you through these processes.
Bringing Value To You
At Epec Engineered Technologies, safety, security and the long term quality of our products are very serious issues. Let us show you how we can bring this value to your medical device.
Power Your Medical Device with Confidence
Partner with Epec for innovative battery solutions and regulatory expertise. Contact us for a safe and efficient power management system!
Request a Quote Request Design Support Request More Information








